
Clinical trial orchestration and data capture in one decentralized platform.

Task
Launch features that are core to Science37's mission of producing a fully decentralized and digital clinical trial platform. Collaborate across the design team, product management, and engineering to create final interface designs.
Process
I led the design experience within our feature pipeline's "workflow" epic. These features included S37's scheduling, user documents, data capture, video calls, legal guardian onboarding, sign-up, and more - any part of the product that had touch-points with our users (Participants, Clinical Research Coordinator, Investigator).
Result
I implemented new design patterns and interactions into the design system and implemented the designs with engineers. The design success had an enormous business impact, bringing in new clients and vital revenue streams that had us meet financial goals.
(Participant) Legally Authorized Representative Login
Managing your permissions as a participant or legally authorized representative for multiple participants across a decentralized clinical trial solution requires a quick, intuitive way to switch between them.

(Participant) Confirming an Upcoming Visit
As a study participant or legally authorized representative, you must view all your required tasks, including confirming upcoming appointments with traveling nurses or a remote call with your clinical research coordinator.

(Participant) Rescheduling a Visit
Sometimes, life happens, and rescheduling is required. Users can quickly jump to their upcoming visit detail page and request a reschedule from their clinical research coordinator. Once approved, the system notifies the participant, and all other parties are informed and rescheduled for that new time.

(Participant) Completing a Daily Task
Participants are asked within the mobile app to complete daily tasks related to their joined study. These tasks may include pain reporting, medication regimens, and other surveys on personal activity.

(Site Staff) Adding a Participant Rep to a Participant
The process of adding participants and any of their possible representatives within a given study can vary within the clinical trial process. In this case, the user could only provide a representative to site staff after being added to the study. So, site staff, in this instance, will add one for them.

(Site Staff) Updating an Upcoming Visit’s Details
During clinical trials, visit parameters sometimes change based on the participant's health or the urgency of the data needed from the participant. Whatever circumstance arises, a clinical research coordinator overseeing the trial must be able to modify the attendees, goal, and timing of the visit to ensure that the study remains within compliance.

(Site Staff) Adding a Form to a Visit
As mentioned above, the coordinator may need to change the criteria of an upcoming visit to meet new requirements or fulfill overdue ones. Making these changes also requires, at times, a means to upload additional forms for the participant or site staff to complete during the visit.

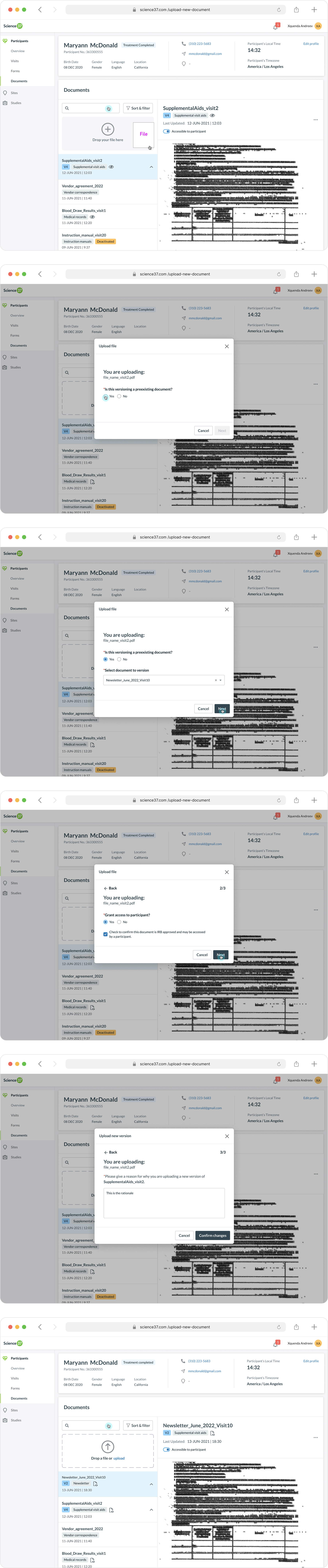
(Site Staff) Uploading Documents to a Study
Proper housekeeping of documents is necessary for a clinical research coordinator. Coordinators must be able to see changes made to documents via themselves or other coordinators assigned to the study and be able to upload, delete, and modify those documents.

Walmart+


Cadillac


The Venetian


Drone Futures









